1997: Established joint venture company named “Government Pharmaceutical Organization- Meriéux Biological Products (GPO-MBP)”, There were 3 shareholders: Government Pharmaceutical Organization, Bureau Crown Property, and Pasteur Meriéux Connaught
2000: Start construction of manufacturing site located at Gateway City Industrial Estate, Chachoengsao
2002: First two Vaccines: Rabies Vaccine and Hepatitis B vaccine were licensed and commercialized for Thai National Immunization Program
2002-2008: Manufactured Vaccines were supplied Thai National Immunization Program under privileged including: Hepatitis B vaccine, Rabies Vaccine, MMR vaccine, Trivalent Influenza vaccine, and DTP-Hep B vaccine. They were under Technology Transferred by Sanofi Pasteur
2006: Manufactured and Licensed Measles Vaccine in Thailand
2007: Develop Live attenuated Live attenuated Japanese Encephalitis Vaccine, recombinant from research to industrial scale
2010: Live attenuated Japanese Encephalitis Vaccine, recombinant was licensed in Australia
2010: Granted WHO Prequalification on Live attenuated Measles Vaccine
2014: Granted WHO Prequalification on Live attenuated Japanese Encephalitis Vaccine, recombinant
2022: 1st December 2022 Substipharm Biologics acquired IMOJEV and Shared of GPO-MBP from Sanofi Pasteur. The company name was changed to Global Biotech Products (GBP) until now
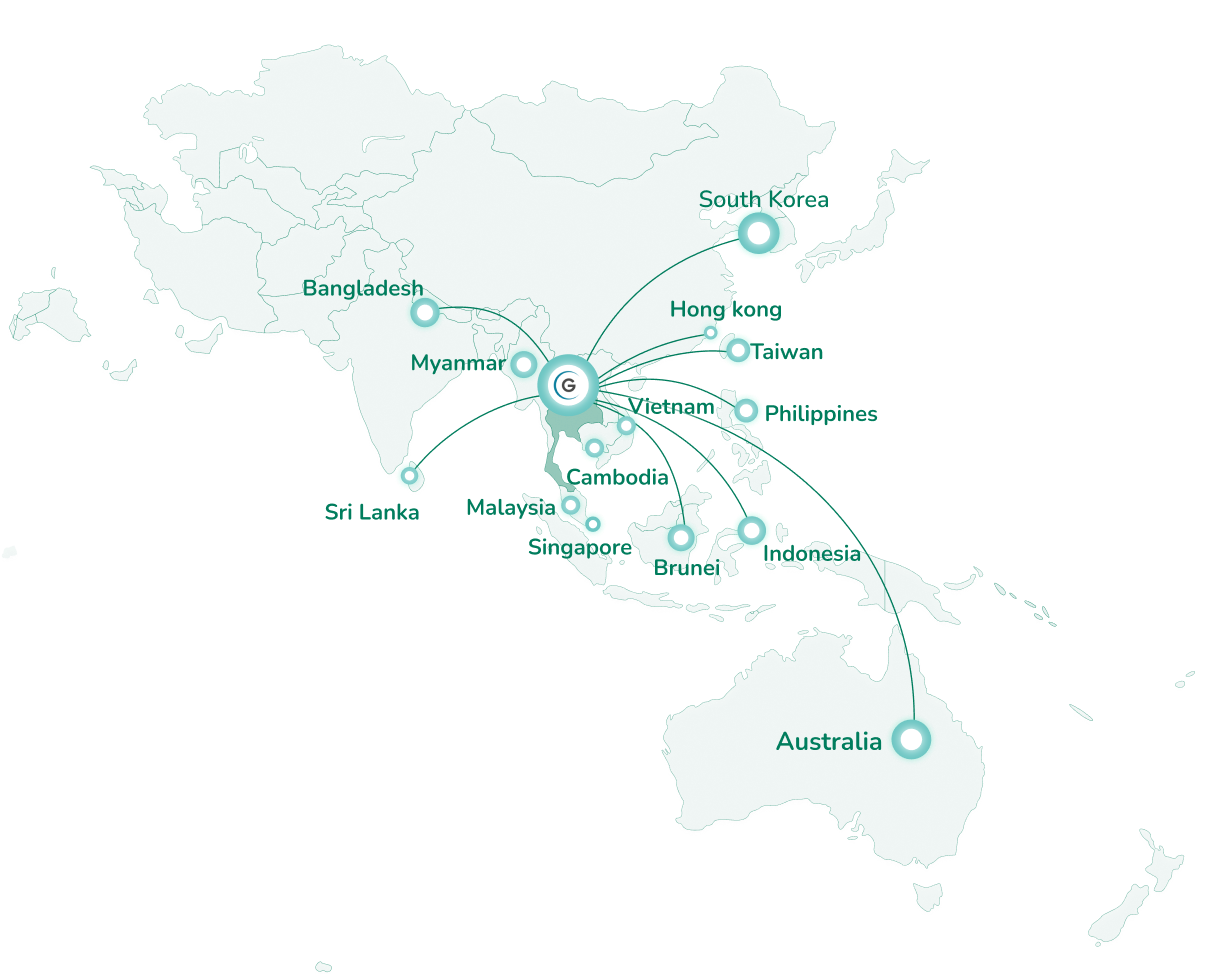
Strongly known for the technical expertise of its people and for the high quality and compliance level, GBP is the unique manufacturer prequalified by WHO and TGA Australian (Health Agency) in Southeast Asian countries. For these reasons the site was chosen to register (country of origin), produce and distribute the Japanese Encephalitis live attenuated recombinant vaccine in the entire region including Australia.
Highly autonomous, GBP demonstrated its ability to manage technology transferred and launch products (11 launches in 10 years) and high level of flexibility of product presentations (1 Dose, 2 Doses, 4 Doses, 10 Doses).



 Company Profile
Company Profile